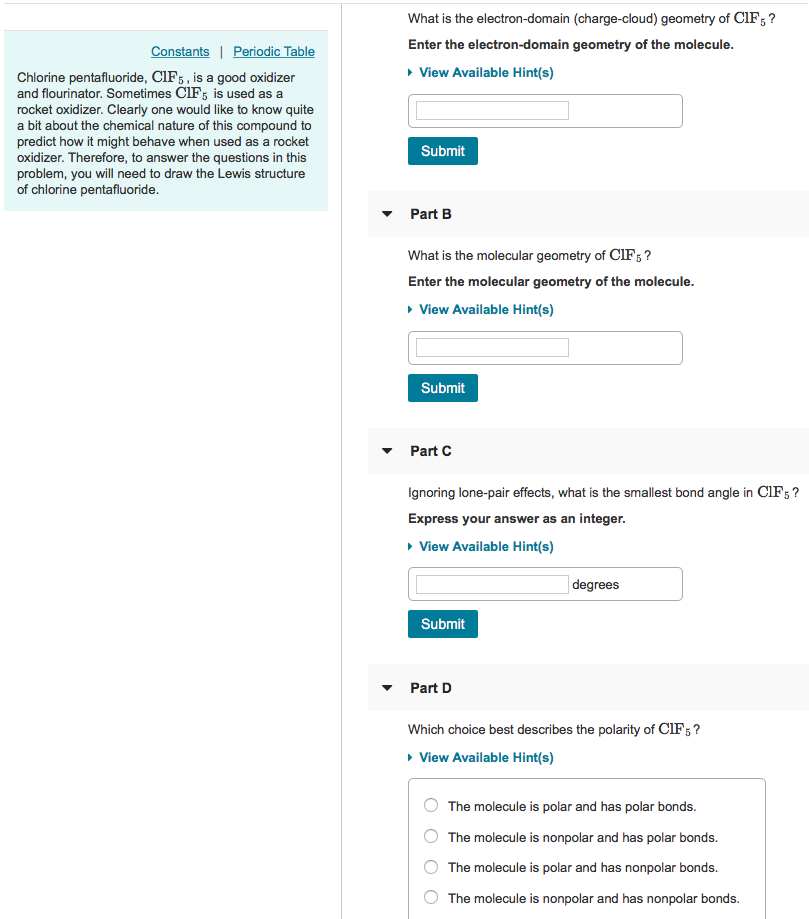
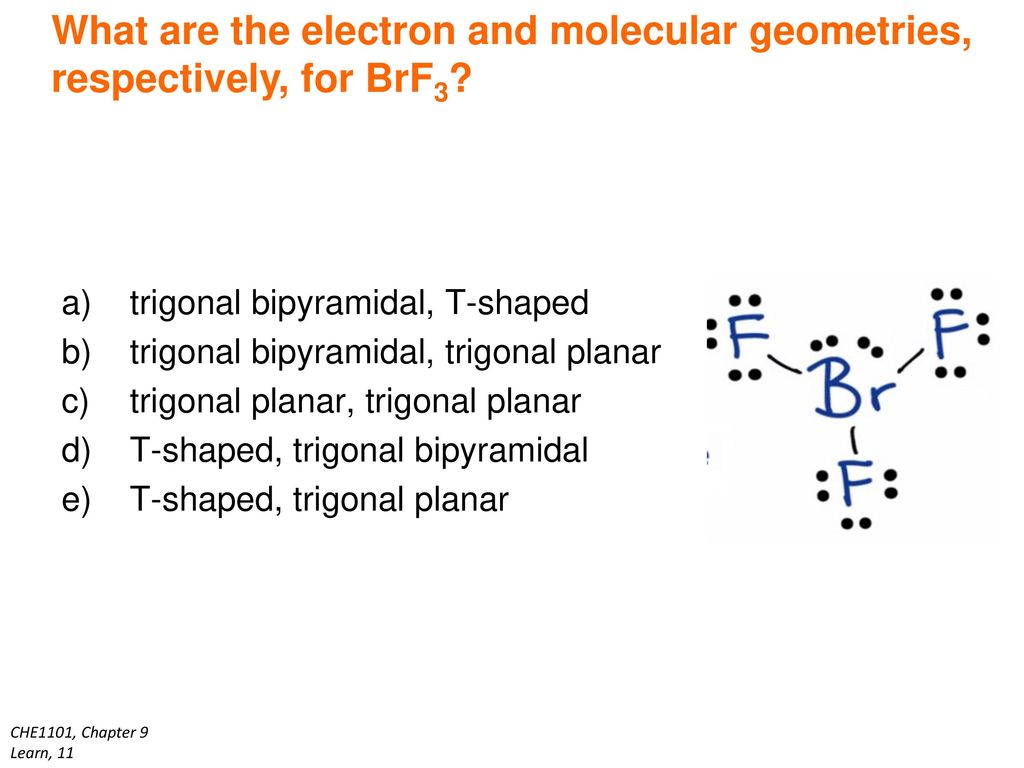
What Is The Electron-domain (charge-cloud) Geometry Of Brf5?
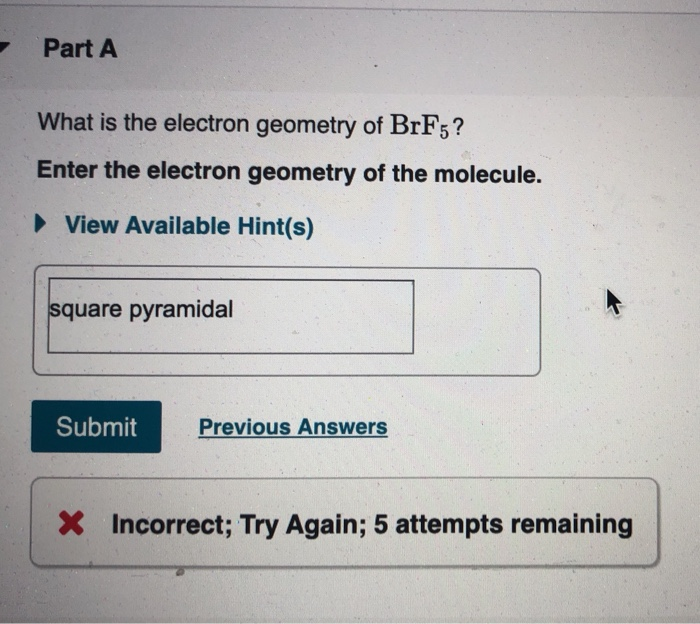
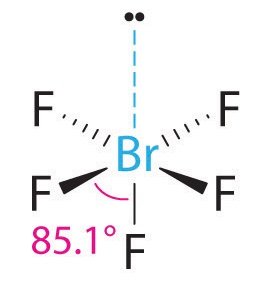
What is the electron-domain (charge-cloud) geometry of brf5?. The bond angles are compressed relative tothose in a perfect octahedron due to the lone pair spreading out more in space than bonded pairs. The molecule will have a total of 36 valence electrons - 7 from bromine 7 from each of the four fluorine atoms and one extra electron to give the ion the -1 charge. 1What is the electron-domain charge-cloud geometry of BrF5.
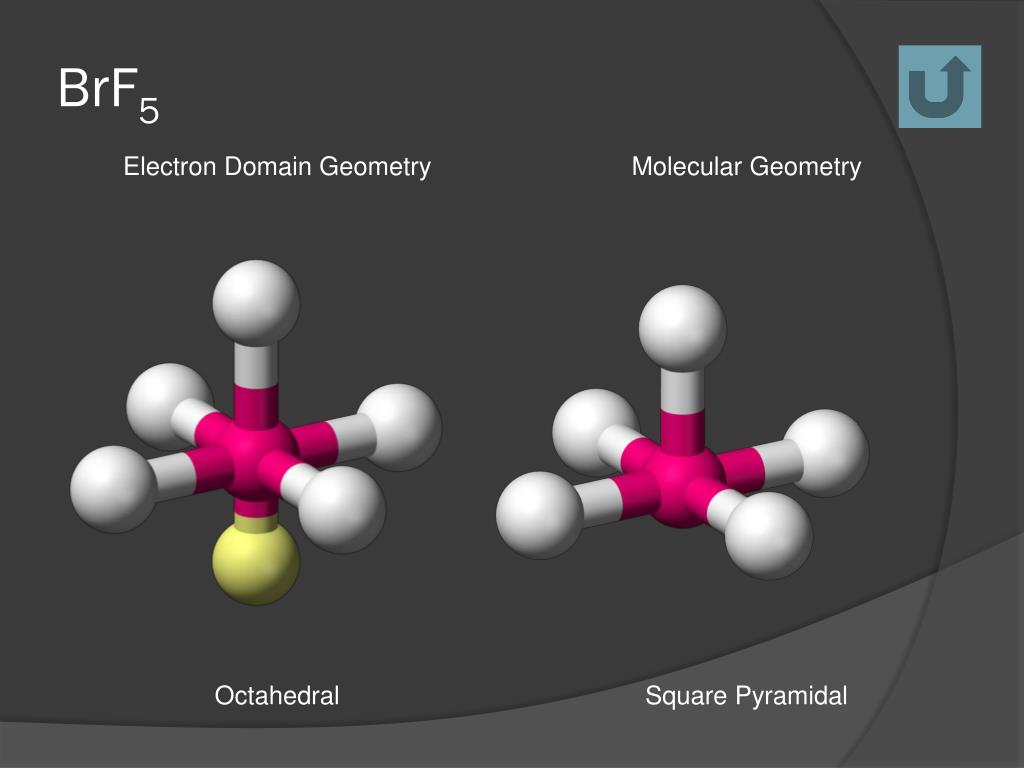
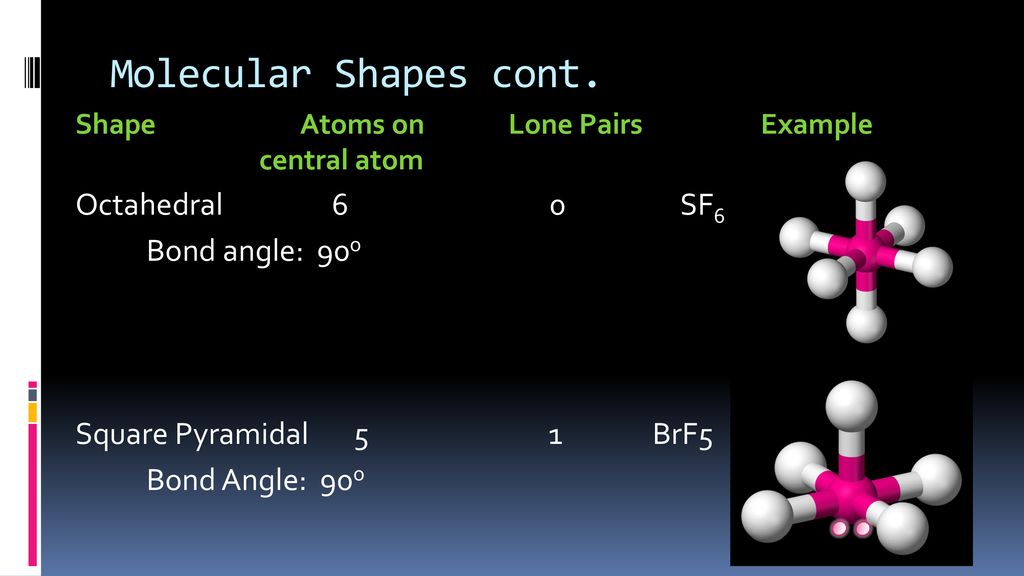
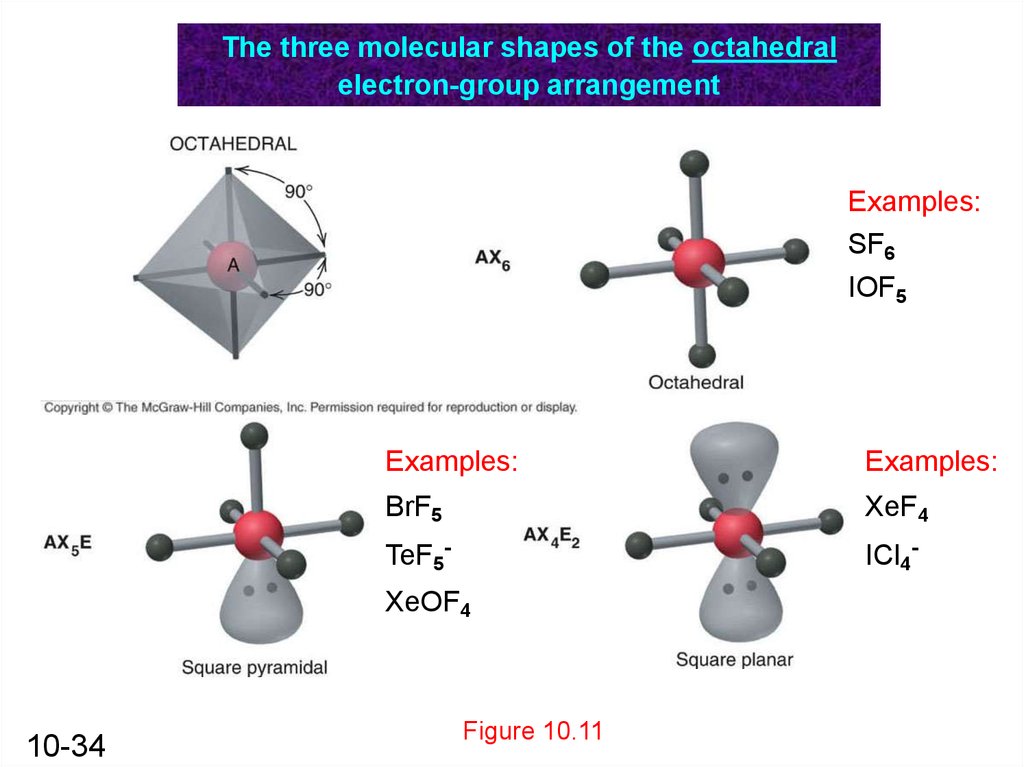
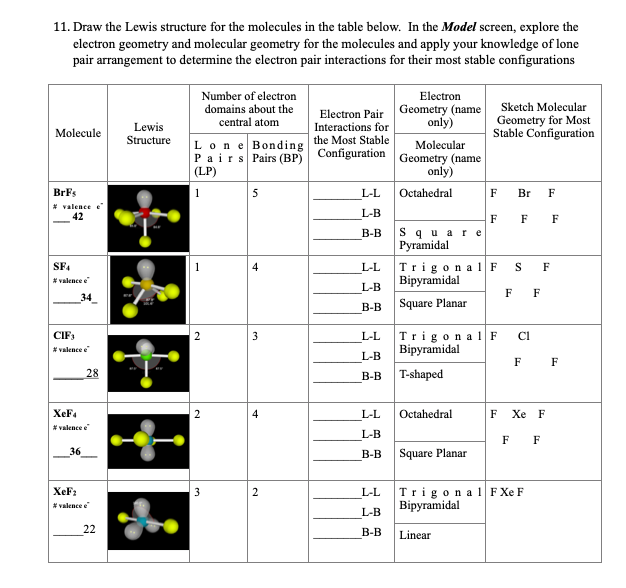
Lewis dot structure of BrF5 Chapter 4 85-105 85. The five fluorine atoms bond to the bromine atom and there is one lone electron pair. The electrondomain chargecloud geometry of BrF5 is octahedral.
BrF5 has been shown to be acutely toxic in animals. Furthermore What is the electron-domain charge cloud geometry of BrF5 Answer and Explanation. What is the electron-domain charge-cloud geometry of BrF5.
What is the electron-domain charge-cloud of BrF5. Express your answer in analogy to the following example. 3Ignoring lone pair effects what is the smallest bond angle in Brf5.
Sp2Part E Which choice best describes the polarity of. This is because the process involves the loss of electrons. Is BrF5 a sp3d2.
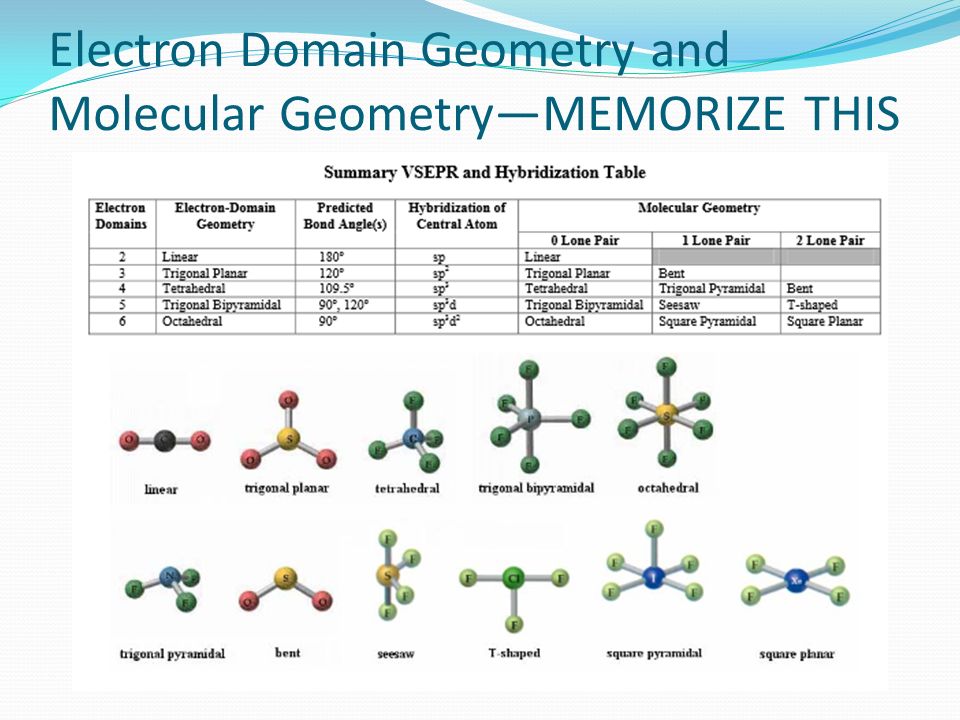
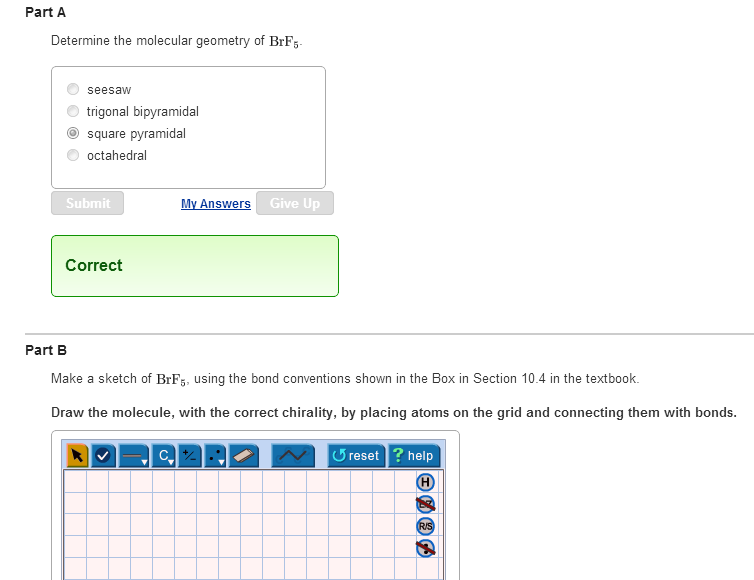
What is the electron domain and molecular geometry of bro2. Hybridisation of BrF5 is sp3d2 with square pyramidal geometry. These symptoms appeared after exposure for a period as short as three minutes.
Which choice best describes the polarity of BrF5. The electron domain and molecular geometry of are tetrahedral and bent or angular respectively.
Octahedral is the edcc geometry and the molecular geometry is.
The electron domain charge cloud geometry of ICI5 s usually positively charged. The electrondomain chargecloud geometry of BrF5 is octahedral. The five fluorine atoms bond to the bromine atom and there is one lone electron pair. The electron domain charge cloud geometry of ICI5 s. The molecule will have a total of 36 valence electrons - 7 from bromine 7 from each of the four fluorine atoms and one extra electron to give the ion the -1 charge. This is because the process involves the loss of electrons. See full answer below. Octahedral is the edcc geometry and the molecular geometry is. Molecular geometry of BrF5.
Part CIgnoring lone pair effects what is the smallest bond angle in BrF5. The five fluorine atoms bond to the bromine atom and there is one lone electron pair. Sp2Which choice best describes the polarity of BrF5. Electron Domain is TetrahedralMolecular Geometry is Trigonal Pyramidal. The electron domain charge cloud geometry of ICI5 s. Furthermore What is the electron-domain charge cloud geometry of BrF5 Answer and Explanation. 3Ignoring lone pair effects what is the smallest bond angle in Brf5.




























Post a Comment for "What Is The Electron-domain (charge-cloud) Geometry Of Brf5?"